Abstract
INTRODUCTION: Chronic lymphocytic leukemia (CLL) with deletion 13q (del13q) has historically better outcome after chemotherapy. However a sizeable number of such patients do progress with a decreased progression free survival (PFS) and overall survival (OS). We analyzed this group of patients treated with FCR to characterize the relationship between clinico-biological parameters and outcome.
METHODS: We retrospectively analyzed 134 CLL patients with del(13q) treated with FCR from March 2004 to August 2014 in our institution. The association between base line characteristics, 12 month minimal residual disease (MRD) status as detected by flow cytometry with PFS and OS was assessed using univariate Cox proportional hazards model on all patients.
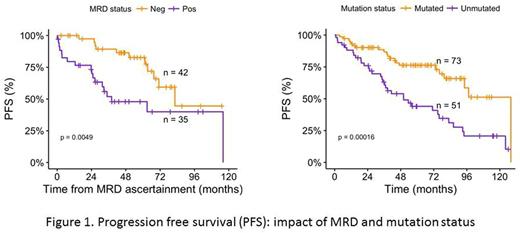
RESULTS: Of 134 patients 63% (n=84) were male and 37% (n=50) female, and median age was 60 (range 35-83) yrs. Median follow up was 70.3 mths (5.9 yrs). Median WBC was 39.3 K/μL (3.2-666.5) and Hb 13.6 (g/dL) (7.1-16.4). Seventy three (54%) were IGVH mutated and 51(38%) unmutated. Eighty nine (66%) had beta-2 microglobulin (β2M) <4 and 44 (33%) > 4 μg/mL. Karyotype was diploid in 107 (80%) patients and abnormal in 16 (12%) patients. By fluorescence in situ hybridization (FISH), %age of cells having del(13q) ranged from 8-98% with median of 55.75%. One locus was involved in 110 (82%) and both loci in 20 (15%) patients. Splenomegaly was present in 55 (41%) and absent in 76 (57%). Ninety six percent (n=129) patients had non bulky disease and only 4 (3%) had bulky disease. After FCR therapy, 83 patients (62%) achieved complete response (CR), 26 (20%) nodular partial response (nPR), 21 (16%) partial response (PR) and 4 (3%) treatment failure. Overall 38% (n=51) patients experienced disease progression. Thirteen percent (n=17) patients died after FCR treatment, of which 8 had progressed and 9 had not progressed. There were 12 malignancies detected after diagnosis of CLL: 4 basal cell carcinomas, 4 squamous cell carcinomas, 1 breast carcinoma, 1 colon carcinoma, 1 small bowel carcinoma, 1 therapy related acute myeloid leukemia, 1 therapy related myelodysplastic syndrome, 1 B-acute lymphoblastic leukemia. Median PFS was 83.1 mths (6.9 years), OS at 5 years was 89.9%, and at 10 years was 79.1%. Median OS was not reached. Of 51 patients who experienced disease progression, there were 8 observed deaths. Due to small number of observed events, estimated survival rates could not be ascertained meaningfully. β2M >4 (HR 1.85, p=0.02), high WBC count (HR 1.55, p<0.01), Hb <10 gm/dL (p=0.01), unmated IGVH (HR 2.78, p=0.001), abnormal karyotype (HR 2.01, p=0.04), splenomegaly (HR 2.45, p=0.001), MRD positivity at 12 months (HR 2.87, p=0.01) had a significant negative impact on PFS. Platelet count <50,000/μl, bi-allelic del(13q) while showing a negative impact on PFS was not statistically significant. Percentage of del(13q) >50% had no impact on PFS. Except for Hb <10 gm/dl (p=0.001), none of other parameters had statistically significant impact on OS. This could be explained by the limited statistical power for detecting OS due to small number of observed patient deaths.
CONCLUSION: In CLL with del(13q), a subset of patients progress after FCR therapy and have poor PFS. β2M, WBC count, Hb, unmated IGVH, abnormal karyotype, splenomegaly, MRD positivity at 12 months have a significant impact on PFS.
Kantarjian: Pfizer: Research Funding; Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Wierda: The University of Texas MD Anderson Cancer Center: Employment; Sanofi: Consultancy, Honoraria; Kite: Research Funding; Karyopharm: Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Janssen: Research Funding; Genentech/Roche: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Acerta: Research Funding; Merck: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Juno: Research Funding. Burger: Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Jain: Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Research Funding; Celgene: Research Funding; BMS: Research Funding; Abbvie: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal